Product Information
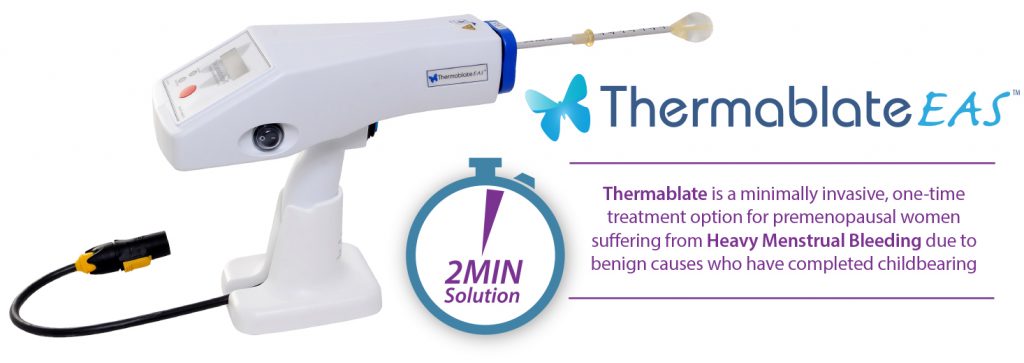
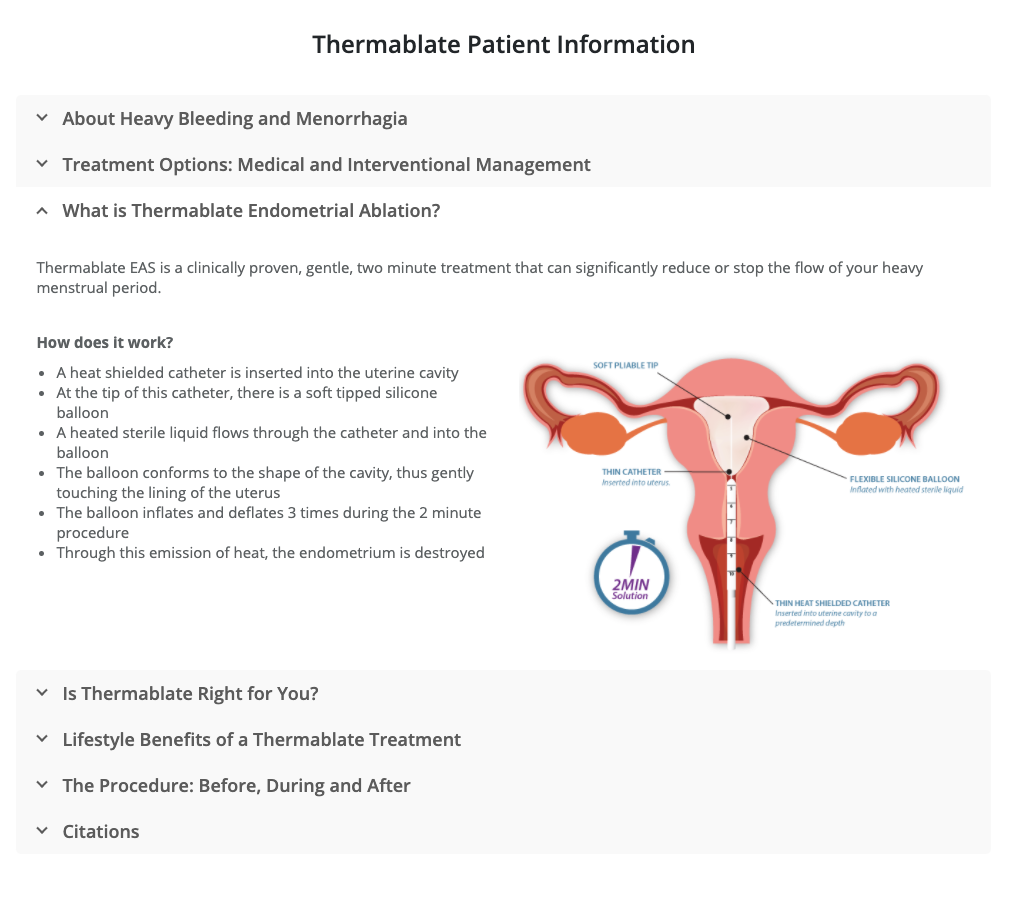
The Thermablate® Endometrial Ablation System is a clinically proven, gentle, two minute treatment that can significantly reduce or stop heavy menstrual periods for women who have completed childbearing.
Performing endometrial ablations with Thermablate® in an Outpatient or Ambulatory Clinic setting rather than as an inpatient treatment, is convenient, cost effective and offers a patient focused solution.
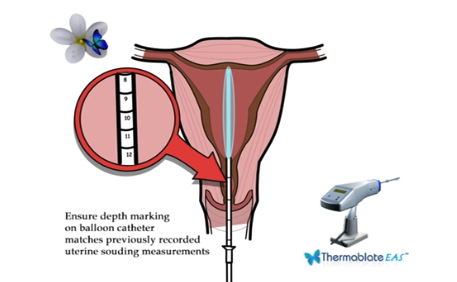
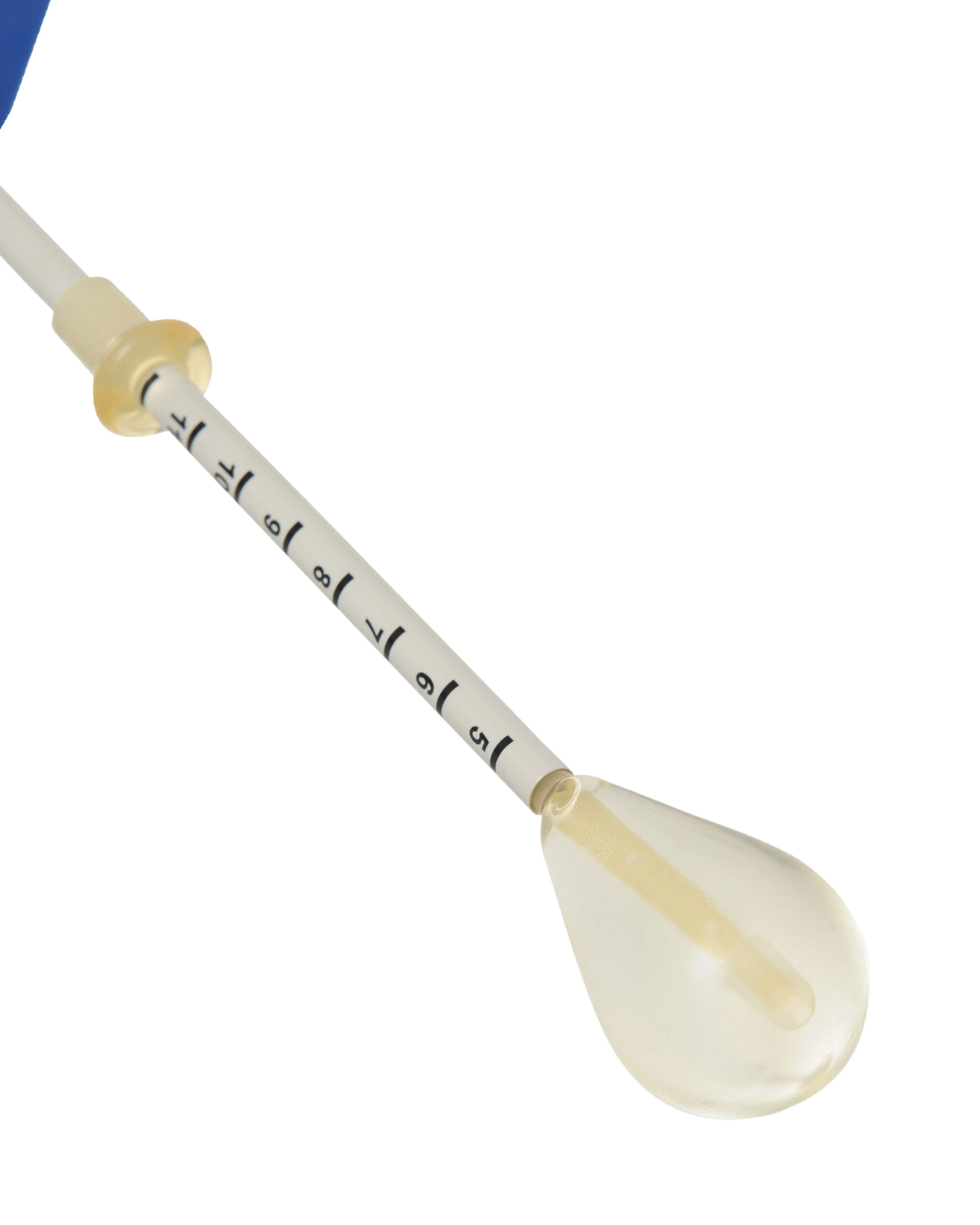
Ensure depth marking on balloon catheter matches previously recorded uterine souding measurements
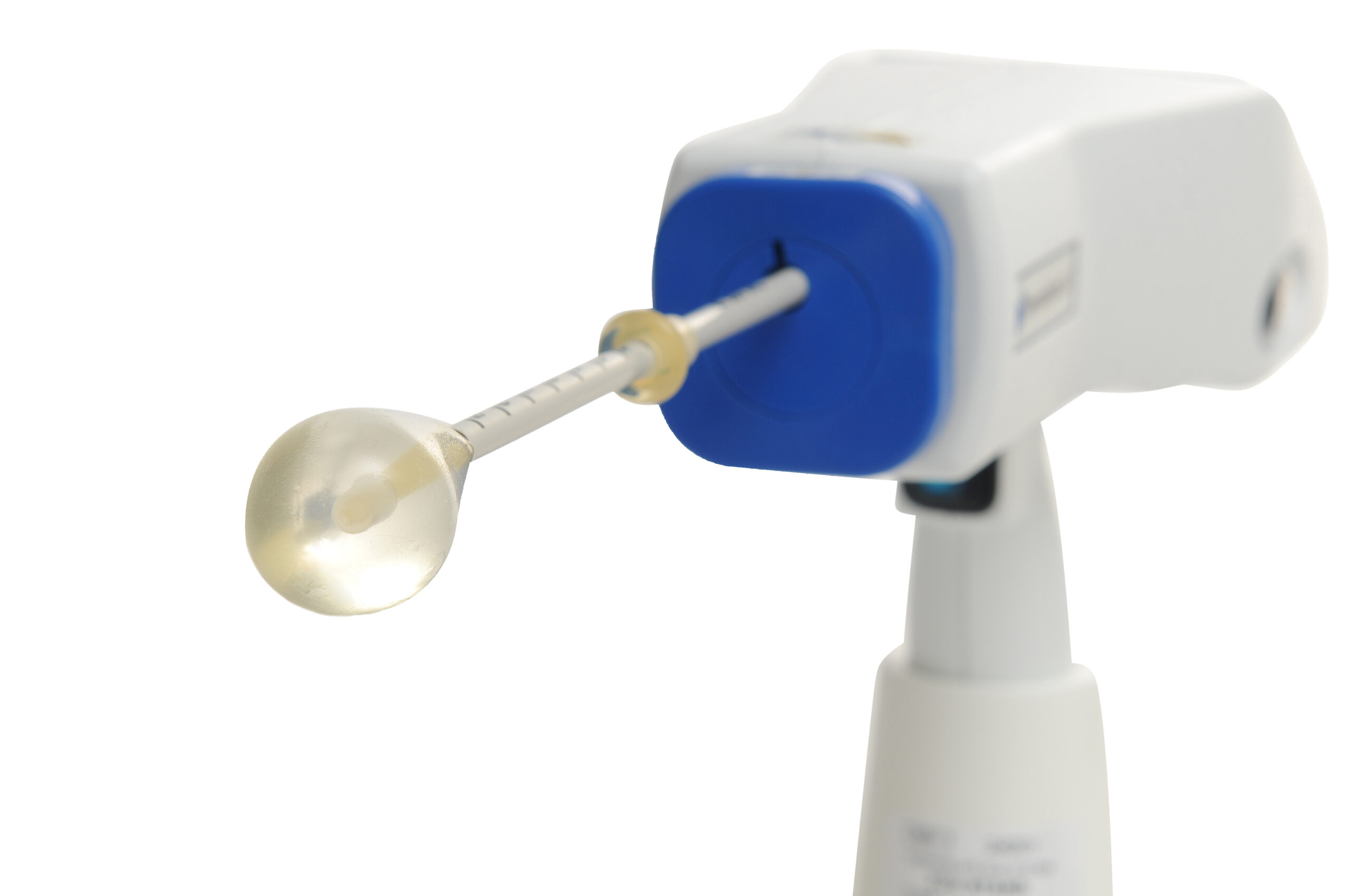
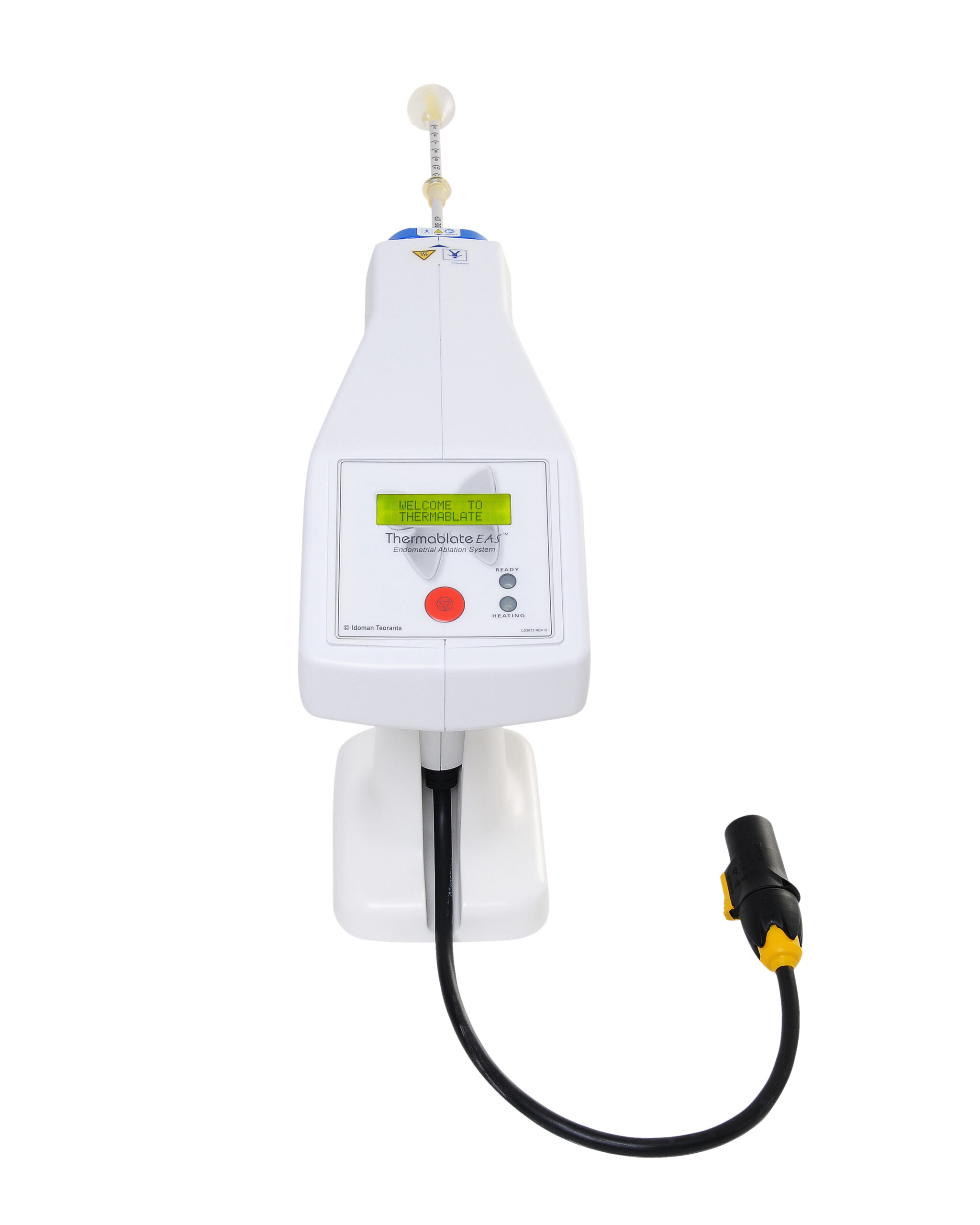
A Thermablate® disposable cartridge is comprised of a slim diameter heat shielded catheter and a soft tipped silicone balloon.
Prior to treatment, the cartridge is connected to the TCU in order to heat the sterile fluid contained in the balloon.
The balloon catheter is gently inserted into the uterine cavity. The TCU automatically transfers pre-heated liquid through the catheter, directly inflating the silicone balloon,
which conforms to the shape and size of most uterine cavities.
Thermal energy is transferred through the balloon, which conforms to the shape and size of most uterine cavities.
Thermal energy is transferred through the balloon, which ablates the endometrial layer of the uterus in two minutes and six seconds.
Advantages:
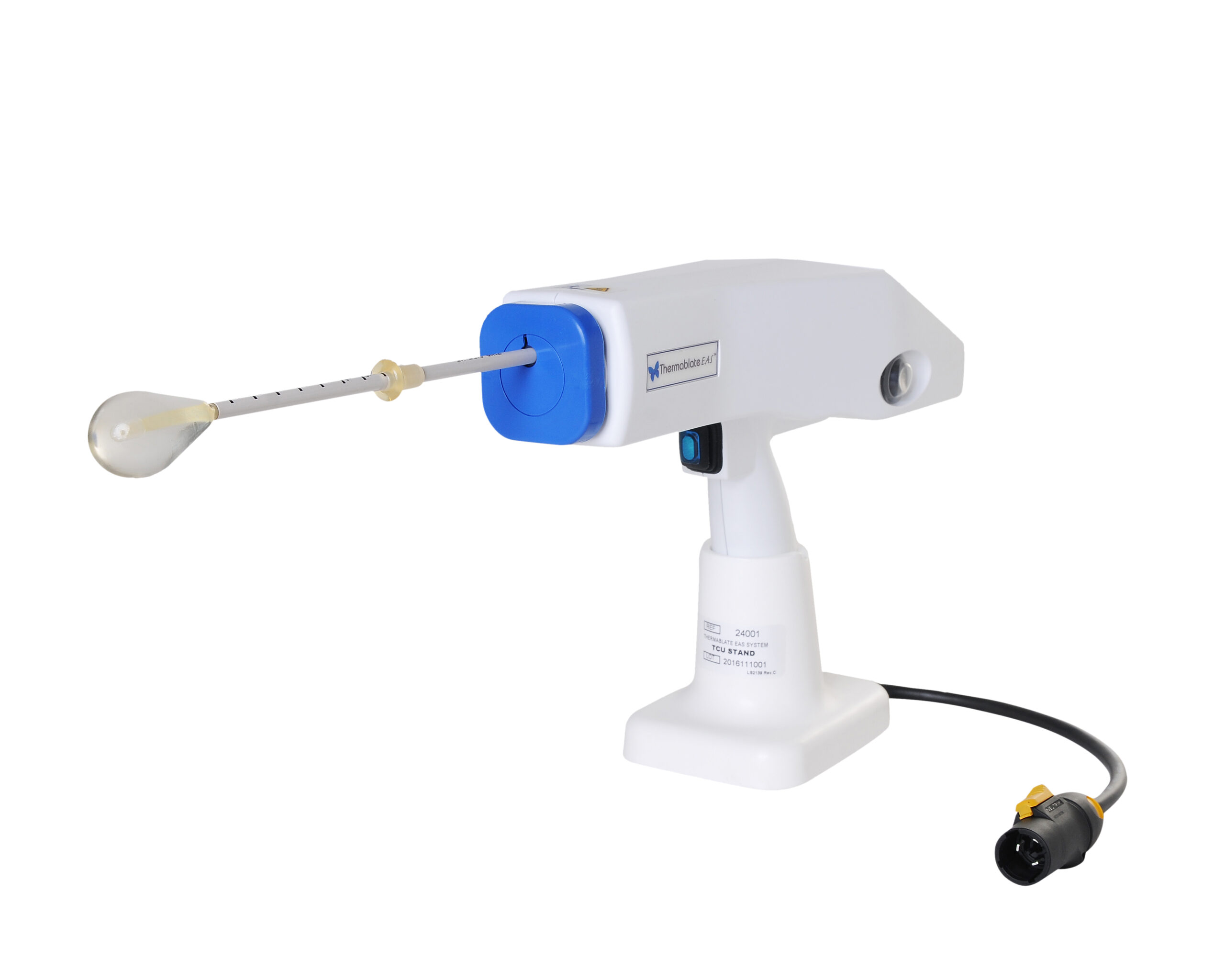
The Thermablate® EAS is made up of a Treatment Control Unit (TCU) and disposable cartridge, often referred to as a balloon catheter.
A TCU is:
Fully automated
Simple to set up
Compact, lightweight
A Thermablate® procedure can be performed under local or general anesthesia with a quick recovery time and no side effects.
Potential Thermablate patients must meet certain criteria to be eligible for treatment:
– Excessive uterine bleeding with no underlying causes
– Completed childbearing
– Pre-menopausal normal uterine cavity with sounding between 8cm and 12cm, inclusive
– Normal endometrial biopsy and pap smear in accordance with Clinical Practice Guidelines
Contraindications
It is important to rule out contraindications prior to treating a patient with the Thermablate system.

Product Specs